Modes of Transmission

Quaternary ammonium compounds (QACs) are cationic surfactants that are both water- and fat-soluble. This allows them to bind dirt and grease in water. In addition to their cleaning properties, some of these compounds also have a germicidal effect and are therefore used as active ingredients in disinfectants [1].
The efficacy of QACs is highly dependent on the molecular structure and comprises vegetative bacteria, yeasts, some fungi, and enveloped and lipophilic viruses [2,3]. In combination with other disinfecting agents, a broader spectrum of activity can be achieved [4]. Such formulations are used, among other things, in instrument and surface disinfection.
The material compatibility of products based on quaternary ammonium compounds can be classified as good. The materials commonly used in the health sector withstand everyday disinfection. This is especially true for floor coverings. The cleaning properties support the maintenance of equipment and objects. Only polymer blends in components subject to heavy mechanical stress should be tested individually for compatibility.
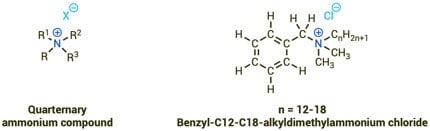
From a chemical point of view, QACs are compounds consisting of a positively charged ammonium cation and a negatively charged anion (see Fig. 1). In the ammonium ion, the central nitrogen atom is bound to four hydrocarbon residues. One of the hydrocarbon residues is often significantly longer than the other residues, usually a chain of > 12 carbon atoms [2]. This long uncharged chain (tail) can interact with other uncharged organic substances, e.g. fats. The positively charged head group, on the other hand, can interact with negatively charged substances [5]. Due to this double functionality, the ability to mediate between charged and uncharged substances, QACs are so-called surface-active substances [5]. These properties are not only responsible for the cleansing effect on surfaces, but also for the disinfecting effect against potential pathogens. By accumulating in the lipid-containing envelope, penetrating through the cell wall, and interacting with the cell components, the pathogens are irreversibly damaged [5,6].
Sources:
1. Bayrisches Landesamt für Gesundheit und Lebensmittelsicherheit. Quartäre Ammoniumverbindungen – Pflanzenschutz- oder Desinfektionsmittel? – Untersuchungsergebnisse 2012.https://www.lgl.bayern.de/lebensmittel/chemie/pflanzenschutzmittel/pestizide_pflanzlich_lm/ue_2012_qav.htm
2. Gerba CP. (2015) Appl Environ Microbiol. 81(2): 464-469.
3. Kramer A und Assadian O (Hrsg.) Wallhäußers Praxis der Sterilisation, Desinfektion, Antiseptik und Konservierung, 2008, S. 770-789.
4. Kramer A et al. (Hrsg.). Krankenhaus- und Praxishygiene, 2016. S. 34.
5. Morrison KR et al. (2019) Tetrahedron Lett. 2019 60(37).
6. Block S (Hrsg.). Disinfection, Sterilization, and Preservation, 1991, S. 250ff.