HARTMANN SCIENCE CENTER

What does virucidal mean?
According to the Centers for Disease Control and Prevention (CDC) in the USA, a virucide is an active agent that inactivates viruses [1]. A disinfectant with this kind of antimicrobial activity acts non-specifically against a virus and, if the disinfectant is used correctly, the virus no longer exhibits infectivity. Virucidal disinfectants play a significant role in infection prevention.
International perspective
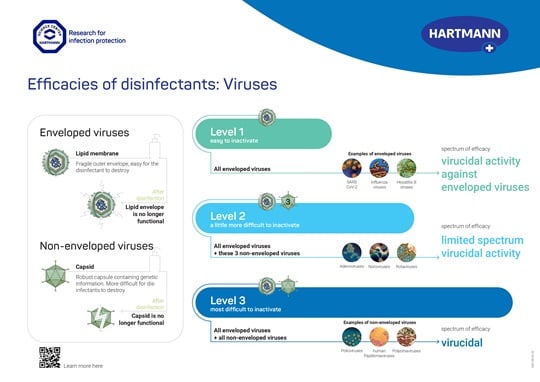
In general, two different kinds of viruses can be structurally distinguished: enveloped and non-enveloped viruses. Enveloped viruses are characterised by an additional lipid coat surrounding the proteinaceous capsule which contains the genetic material. Enveloped viruses are usually easier to inactivate compared to non-enveloped viruses, because the viral envelope can serve as a point of attack for the disinfectant.
In their respective guidelines regarding hand hygiene, the CDC [2] and the World Health Organization (WHO) [3] consider the structural properties mentioned above for their recommendations. Therefore, the CDC and the WHO discriminate between ‘virucidal activity against enveloped viruses’ and ‘virucidal activity against non-enveloped viruses’. However, in other countries or jurisdictions different classification systems for virucidal activity exist. For instance, in the European Union an intermediate level of activity against enveloped and certain non-enveloped viruses exists [4].
‘Virucidal activity’ can be demonstrated according to different norms
When referring to declarations of specific spectra of efficacy it is important to give the respective test method or norm. Depending on the country or region, different standardised test methods, according to which virucidal activity can be demonstrated, are available. The test methods differ, among other things, with respect to the test pathogens that represent specific groups of microorganisms.
Proof of ‘virucidal activity’ according to the European norm DIN EN 14476
At the European level, virucidal activity is demonstrated according to EN 14476. The Technical Committee 216 (TC 216) “Chemical disinfectants and antiseptics” established a three-phase model for standardised testing of antimicrobial activity. In this model, EN 14476 is a Phase 2/ Step 1 method. This means that a quantitative suspension test is used to test a disinfectants efficacy against specific test viruses under laboratory conditions [5]. The suspension test examines how the disinfectant acts on the test pathogens with the addition of an organic load (e.g. protein, blood). After mixing of test pathogens and organic challenge, and a waiting of a defined time, an aliquot of the mixture is used for further analysis. In order to be effective within the virucidal spectrum of activity, the disinfectant must inactivate 99.99 % of test viruses (4 log reduction) [6]. EN 14476 is tested with the following test pathogens for the claim ‘virucidal activity’:
•Adenovirus
•Murine norovirus
•Poliovirus
•Murine parvovirus (in chemo-thermal disinfection)
Sources: